The global leader in equipment for inhaled product testing
Copley Scientific is the world’s leading manufacturer and supplier of inhaler and nasal drug product test equipment with a comprehensive range of products for testing:
• Metered dose inhalers (MDIs), dry powder inhalers (DPIs), nebulisers, soft mist inhalers (SMIs), nasal sprays, nasal aerosols and nasal powders products
• Both innovator (novel) and generic drug products
• In accordance with the USP/Ph.Eur./FDA/EMA requirements
• To secure better in vitro-in vivo correlations (IVIVCs)
• Productively within a high-throughput environment.
Trusted equipment for routine testing
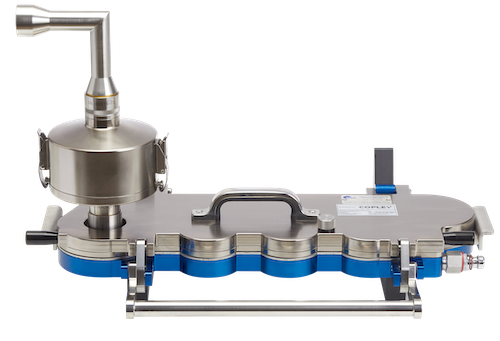
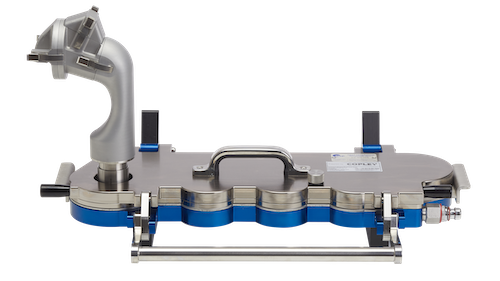
Copley equipment and accessories are the mainstay of the majority of inhaled product testing facilities. Robust, well-engineered solutions that fully meet the pharmacopoeia specifications for Delivered Dose Uniformity (DDU) and Aerodynamic Particle Size Distribution (APSD) include:
• Dose uniformity sampling collection devices for MDIs, DPIs, nebulisers, SMIs and nasal drug products
• Next Generation Impactor (NGI), Andersen Cascade Impactor (ACI), Multi-Stage Liquid Impinger (MSLI) and Glass Twin Impinger (GTI) for APSD measurement
• All the ancillaries and accessories required to efficiently implement a standard inhaler testing set-up where required: mouthpiece adapters, critical flow controller, vacuum pump, breath simulator, flow meter, tubing, connectors and validation tools
• Drug monograph specific equipment for testing Albuterol, Fluticasone Propionate/+ Salmeterol
APSD Data Analysis Software: Inhalytix®
Inhalytix is a fully compliant data analysis software package offering analysts a flexible and validated solution for the analysis and reporting of the APSD drug output from all inhaled devices in accordance with pharmacopoeial and regulatory requirements.
Innovative tools for better IVIVCs
Working closely with industry experts and academics, Copley has an established track record of successful innovation to meet evolving testing needs.
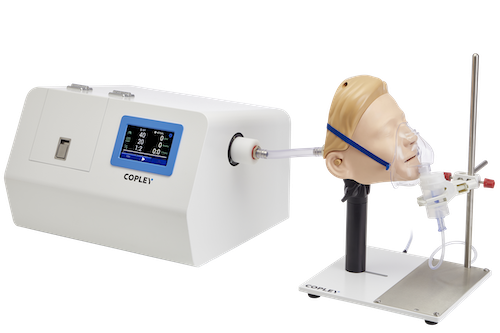
Better IVIVCs have the potential to accelerate time to market for drug development. They also underpin the secure demonstration of bioequivalence – a critical step in the development of generic OIPs – and help reduce the risk of unexpected outcomes in clinical trials. The set-up shown opposite uses new components alongside established equipment to deliver data that represent in vivo behaviour more accurately than data from the standard test methodologies originally designed for QC. It includes a(n):
• Alberta Idealised Throat (child or adult) for a realistic representation of the human mouth/throat geometry (or Alberta Idealised Nasal Inlet for a realistic representation of the adult nasal cavity for nasal drug product assessment)
• Breathing simulator for assessing how different patient representative flow profiles impact drug delivery performance
• Mixing inlet to decouple the variable flow rate through the inhaler from the constant sampling flow rate, when using a cascade impactor
• Equipment for measuring drug dissolution rates specific to size fractions within the inhalable aerodynamic particle size range
Tools for automation
Inhaler testing is widely recognised as a labour-intensive process. Copley supplies a broad range of labour-saving devices and automated solutions supporting both sampling and recovery for DDU testing and APSD measurement. By reducing manual input, these tools also help to eliminate the risk of repetitive strain injury (RSI), while at the same time boosting test accuracy and productivity for both R&D and QC. Furthermore, Copley also supplies a range of abbreviated impactors, which, when combined with Efficient Data Analysis (EDA) techniques, can also be used to enhance productivity and accelerate product screening.
EnviroMate ™ – Benchtop environmental chamber
Both the Ph.Eur. and USP emphasise the importance of controlling environmental conditions, especially when temperature and/or humidity limits are indicated on product labels or specified in relevant monographs. It’s considered best practice to extend such environmental controls to all DDU and APSD testing applications to reduce variability and enhance the accuracy, sensitivity, and reproducibility of data. EnviroMate™ offers a practical solution for analysts seeking stable environmental conditions. This cost-effective, compact, benchtop environmental solution aids analysts in achieving stable environmental conditions, maintaining uniform temperature and humidity distribution throughout the chamber. Additionally, the built-in anti-static system minimises the effects of electrostatic charge, providing analysts with consistent environmental control in the immediate test area and enhancing OINDP test data accuracy and repeatability.
The reassurance of exemplary customer support
Copley Scientific is a company with a focus on quality and a reputation for delivering valued, cost-efficient testing solutions to the pharmaceutical industry that extends over many decades. Covering the complete inhaler testing range is a guarantee of exemplary service and support that extends to:
• Highly informative brochures, user manuals and articles that explain how to use equipment to achieve successful testing within the regulatory framework
• A comprehensive range of fully customised in-house and on-site service contracts to provide quality maintenance and calibration at a competitive price
• In–depth technical and application training from globally recognised experts
• Qualification tools and a complete, quick turn-around stage mensuration service to maintain validated impactor performance
Read white papers
An Introduction to Cascade Impaction
Understanding the links between drug delivery route and in vitro test methods
Using Breathing Simulators to Enhance Inhaled Product Testing
For further information, click here.