FLUIDDA is the leading R&D company in the field of quantitative image analysis. Its proprietary technology – Functional Respiratory Imaging – offers a unique entry point into personalized medicine for patients suffering from respiratory diseases and sleep-related breathing disorders, by providing patient specific imaging biomarkers.
Implementing FRI in early clinical research results in a cost-effective and time-saving screening of the most promising respiratory drugs.
FRI PROVIDES ‘FIRST IN CLASS’ PATIENT SPECIFIC BIOMARKERS
FRI is a unique combination of CT images with advanced engineering tools, developed and commercialized by FLUIDDA. FRI produces patient specific 3D airway and lung geometry defining regional airway resistance as well as patient specific aerosol deposition patterns.
FRI results in highly clinical relevant patient specific biomarkers, measuring:
- Internal airflow distribution
- Lung volume
- Lobe volume
- Airway volume
- Airway resistance
- Lobar perfusion
- Aerosol deposition
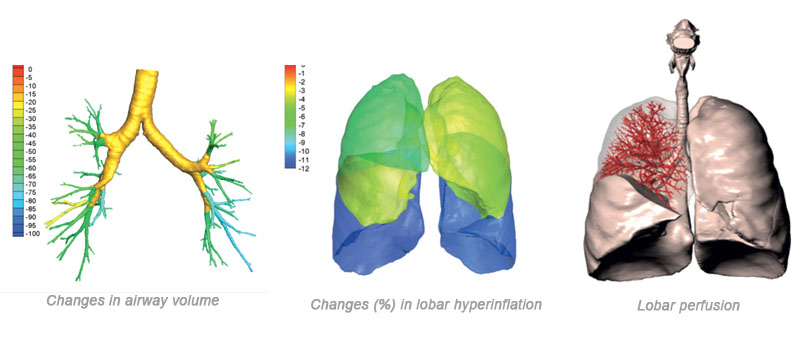
Imaging biomarkers
Proven FRI benefits include:
- FRI identifies the most promising respiratory drugs in a cost and time-effective way.
- FRI reduces the number of patients in the clinical trial by a factor 3-8 while preserving statistical significance.
- FRI helps identify ineffective therapies sooner (fail fast – fail often).
- FRI speeds up the registration process of compounds in the therapeutic area of respiratory diseases.
FRI reduces clinical trial costs
FRI dramatically reduces the cost of clinical trials. The development cost of a respiratory drug, at $1.6 billion per registered product, is significantly higher than other medications. FRI reduces trial costs in two ways. Its highly sensitive outcome parameters make it possible to reduce the number of subjects required for a well-powered study. And, since FRI uses an approach that integrates both pre-clinical and clinical activities, it helps optimize the expensive translational process.
The benefits of FLUIDDA’s proprietary FRI technology have been validated in over 20 clinical trials involving a total of 750 patients performed in collaboration with various academic and medical research centers. The trials confirmed the higher sensitivity of our novel FRI approach over that of existing standard diagnostic tests such as spirometry. This is illustrated by our peer reviewed publications listed on www.fluidda.com