What is a Digital Biomarker and Why Is It Important?
Date: September 10, 2021
Time: 9:00 – 11:00 AM US ET
Moderated by: Marta Lombardini, Device Development Manager, Chiesi Group and Lee Nagao, Senior Director Science, Regulation & Policy, IPAC-RS Secretariat
The first in an IPAC-RS webinar series on digital inhaled drug delivery devices, this webinar includes two parts: “Realizing the Vision of Digitally Enabled, Patient-Centric Clinical Trials” with Marissa Dockendorf and Bryan Hansen of Merck Research Laboratories and “The Progression of Digital Biomarkers in Clinical Trials and Beyond” with Joe Corrigan and James Blakemore of Cambridge Consultants.
Register for the webinar here
Smart Innovation through Hybrid and 505(b)(2) Regulatory Pathways
Date: June 10, 2021
Time: 9am – 11am EDT
The Hybrid and 505(b)(2) regulatory pathways can allow pharmaceutical companies to bring improved medicines onto the market while alleviating some of the cost and time associated with traditional innovative development. Join a panel of industry experts from Lonza, Camargo, IMA Active, J2F Pharma and University of Bonn at our next Virtual Innovation Day for a deep dive into case studies and actionable insights for navigating pharmaceutical regulation with innovation.
Register now for this free event
Understanding the impact of surface properties on quality and performance of high-dose dry powder inhaler formulations
Date: January 8, 2021
Time: 8 am PST, 11 am EST, 5 pm CET
Presented by: Qi Tony Zhou, Associate Professor in the Department of Industrial and Physical Pharmacy at Purdue University
Qi Tony Zhou will discuss “Understanding the impact of surface properties on quality and performance of high-dose dry powder inhaler formulations” in this webinar presented by the International Society for Aerosols in Medicine (ISAM). The webinar is open to all ISAM members and may be particularly useful for early career / young researchers as well as clinicians.
For ISAM membership information, click here
Webinar link: https://zoom.us/j/97589941381?pwd=b1VoS3g4VFowc0Z4dmdLTlUyaUN5Zz09

A CONVERSATION WITH THE FDA: Perspectives in the Time of COVID-19 (IPAC-RS)
Date: November 2, 2020
Time: 10:30 a.m. EST
Moderator: Mary Devlin Capizzi, IPAC-RS Secretariat and Legal Counsel
Panelists: Richard Lostritto, FDA; Brian J. Hasselbalch, FDA; Carla Vozone, Hovione; Martin J. Oliver, Vectura
The IPAC-RS Regulatory Roundtable Series is focused on bringing together leaders in the Orally Inhaled and Nasal Drug Product (OINDP) space. Invited guests will include regulators, industry thought leaders, business leaders, academics, patient groups and others who comprise the OINDP “ecosystem.”
Register here

Translating Inhaled and Nasal Technologies For The Delivery of Biologics
Date: October 14, 2020
Time: 14:00 BST
Presented by: Mark Parry, Intertek Melbourn Technical Director
Inhaled and nasal delivery has specific advantages as a delivery platform outside of its traditional use for asthma/COPD and seasonal rhinitis/sinusitis – it can offer real advantages for the delivery of therapeutic biologics.vDuring this short presentation, Intertek’s Technical Director, Mark Parry, will provide an overview of current technologies available and successfully marketed products with a look at the challenges to development, and solutions available, when formulating for these delivery routes. Mark will also describe key considerations when rapidly repurposing existing products for inhaled delivery.
Register at: https://www.intertek.com/pharmaceutical/fop-webinar-melbourn-translating-oindp-biologic-delivery/
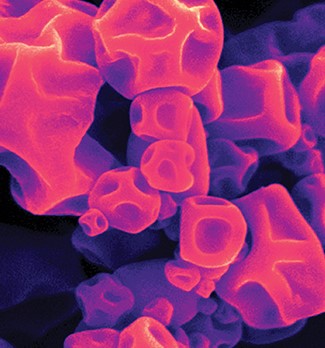
Technical considerations for capsule-based inhalation product development
Date: on demand
Presented by: Herbert Chiou, Product Development Lead and Salvatore Mercuri, Head of NPI & MSAT, Lonza Pharma & Biotech
Drug delivery to the lung is an important and growing field of research for both local and systemic treatments. Efficient nasal and pulmonary drug delivery requires precise particle size and density control. Lonza Pharma & Biotech has premier particle engineering capabilities with which to meet the exacting formulation requirements for dry powder inhaler (DPI) applications, utilizing both spray drying and particle size reduction technologies. These capabilities extend to the ability to control particle properties to interact positively with both capsule and delivery devices, making Lonza a preferred service partner for DPI products.
Webinar link: https://go2.lonza.com/SV-200407-WBN-SMM-1313-INH-Capsule-based-product-development_01-Registration-page.html
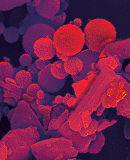
Engineering approaches to respiratory drug delivery: Mannitol case study
Date: on demand
Presented by: Cameron Kadleck, Respiratory Scientist, Product Development, and Jimmy Beaty, Respiratory Scientist, Product Development, Lonza Pharma & Biotech
Both spray drying and jet milling are commercially viable engineering processes for the development of respirable drug products. This case study explores the material and performance properties of mannitol, spray-dried and jet-milled, for respiratory delivery. The head to head comparison reveals opportunities and risks for designing a product based on each approach.
Webinar link: https://go2.lonza.com/SV-200730-WBN-SMM-1314-INH-Engineering-Approaches-to-Respiratory-Drug-Delivery_02-View-Webinar.html

A Quick Guide to IVIVC Testing
Date: Wednesday 15 July
Time: 10:00 AM – 11:00 AM BST
Presented by: Anna Sipitanou, Copley Scientific Business Development Manager
Better in vitro-in vivo correlations (IVIVCs) have long been an industry aim, but the current climate clearly adds impetus to the desire for progress. Inhaled product development in particular presents some unique challenges in this respect. The difficulty of precisely correlating drug deposition behaviour with clinical efficacy, the impact of patient-to-patient variability and the complex interaction between formulation and device, all complicate the development process. This webinar will cover the basics of inhaled product IVIVC testing and will be examining the difficulties and challenges that analysts might face.
Register at: https://meeting.zoho.eu/meeting/registersessionId=1237351124

ISAM webinar: Breathing is enough – Spread of SARS-CoV-2 via Aerosols and the Consequences for Infection Control
Date: Monday 20 July
Time: 11:00 AM – noon EDT
Presented by:GS-Bio-Inhalation CEO Gerhard Scheuch
More on Gerhard Scheuch’s recent letter to the editor of the Journal of Aerosol Medicine and Pulmonary Drug Delivery titled “Breathing Is Enough:For the Spread of Influenza Virus and SARS-CoV-2 by Breathing Only.
ISAM webinars are open to all ISAM members. Membership information available here
Webinar link: https://univ-tours.adobeconnect.com/isam/


