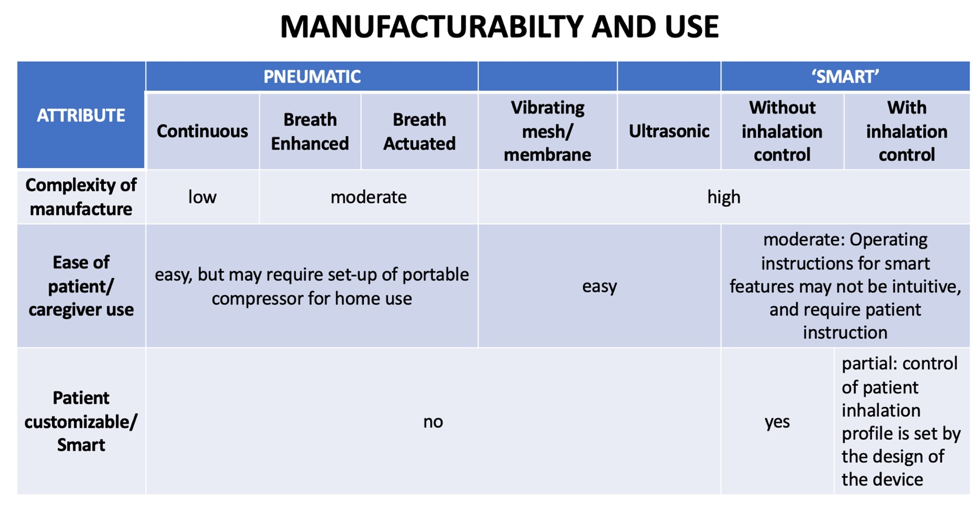
Vibrating mesh/membrane and ultrasonic nebulizers are also generally easy for patients to use but offer no customizable features. Smart nebulizers with or without inhalation control may require patient instruction because the operation of the smart features may not be intuitive. Smart nebulizers without inhalation control are generally fully customizable by the patient to the extent allowed by the associated electronics; smart nebulizers with inhalation control may allow for some customization; however, control of the patient inhalation profile is set by the design of the device to optimize drug deposition.
Other factors that might affect cost
Nebulizers as a combination of device and medication
The focus of this article is on the properties of the various classes of nebulizing system presently available to clinicians, and the qualitative price guide, of necessity, is based on the device component only. It is recognized that where a particular nebulizing system is paired with a specific drug product, as happens where the indication is limited to that particular device-drug combination, the cost to the patient will likely be greater than is the case for a general-purpose or so-called ‘universal’ nebulizer. This difference arises because of additional regulatory requirements for the paired device-drug product, amongst other issues that are beyond the scope of this article to define.
In the US, the regulatory situation for nebulizers as a class of OIP has become more complicated in recent years. Specific nebulizers paired with specific APIs, often because of safety considerations (e.g. high potency, potential for side-effects at low doses) are referred to as drug-specific nebulizers, and are treated as combination drug-plus-device products by the Food and Drug Administration. On the other hand, general purpose (“universal”) nebulizers can be registered as stand-alone medical devices. General purpose nebulizers are prescribed separately with a wide range of different APIs commonly prescribed for chronic diseases such as asthma and chronic obstructive pulmonary disease (COPD) and deemed suitable for use, based on clinical experience.
In Europe and Canada, mostly harmonized regulatory guidance for nebulizer quality control was issued in 2006 by the European Medicines Agency and Health Canada. This guidance reflected the dependency of safe nebulizer use on the selected formulation/device as a combination. Under the 2017 European Union Medical Device Regulations, nebulizing systems that can be used to deliver different formulations (equivalent to general purpose nebulizers), or those devices that are not fully integrated with the drug product, are regulated as medical devices.
Portability
The portability of a nebulizing system is an attribute that may be of concern to clinicians and patients. The situation is complex for pneumatic nebulizers, because these systems are used in widely differing environments, ranging from the emergency room and floors of a hospital, where piped compressed gas supplies are available to the domestic environment, in which a portable compressor will be needed. Furthermore, in recent years, improved miniaturized designs of portable compressor have become available, which greatly improve portability in environments where such a power source is needed.
The portability of nebulizers requiring electronics in order to function, which comprise all the other classes, depends critically on the size and weight of the controller, as well as the need for household mains- or battery-derived electric power. It is therefore not possible to provide generalized statements regarding portability for each class of nebulizing system, as the individual design together with the environment of intended use will dictate how portable a particular device will be.


