Q: What new testing equipment is needed to meet the requirements of this new chapter?
A: Chapter <1602> requires the application of patient-relevant breathing profiles during all ED measurements, which can be achieved using a breathing simulator and a suitable filter holder (Figure 2). For the testing of facemask equipped spacers and VHCs, a facemask test apparatus that applies the facemask to the face model under the appropriate conditions of force and angle (figure 1) can facilitate analysis using the ED test methods described in USP <1602>.
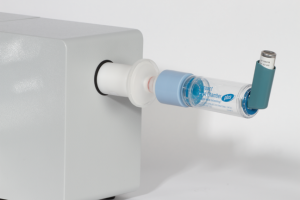
Figure 2: Typical system set-up for testing emitted dose (ED) of MDIs with add-on devices utilizing a mouthpiece
A regular mouthpiece adapter can be used for APSD measurements when interfacing spacers/VHCs that use a mouthpiece to a cascade impactor; however, interfacing spacers/VHCs when a facemask is used is much more challenging.
The pharmacopeial method suggests removal of the facemask before connecting the spacer/VHC to the impactor to ease testing and to avoid the introduction of an ill-defined, unrepresentative connection. However, commercial products that enable direct connection to the impactor via the same face models used in ED testing are now available (Figure 3).

Figure 3: Commercial solutions have been developed to enable direct interfacing of a spacer/VHC with facemask to cascade impactors, and timed flow control, for APSD testing
These products provide a well-defined interface in terms of dead space representation, easing connection of the spacer/VHC to the impactor and making it feasible to gain clinically relevant data with a facemask in place for both ED and APSD testing.
A suitable vacuum pump used in conjunction with a breath actuation controller and an MDI actuation sensor can precisely control time delays between MDI actuation and the onset of flow through the impactor, improving the accuracy and reproducibility of the method (Figure 3).


