Q: Some spacers and VHCs use facemasks instead of a mouthpiece. How does Chapter <1602> take account of these?
A: The purpose of testing facemask-based devices is to confirm that the ED from a spacer or VHC used together with a facemask is equivalent to the ED obtained in the fully-coordinated scenario with the facemask removed.
ED testing for devices used with facemasks is performed in the same way as for a spacer or VHC used with a mouthpiece, except that a face model is introduced between the facemask and collection filter. In order to achieve realistic dead space, the chapter requires the use of age-appropriate face models and, to ensure that the facemask seals to the face model in a clinically appropriate way, a force of 1.6 kg (or a suitably validated value) must be applied to the facemask (Figure 1).
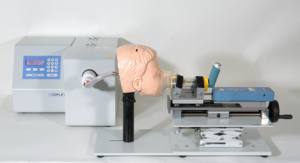
Figure 1: Emitted dose (ED) equipment for testing spacers and VHCs in conjunction with a facemask
Naturally, as technology has progressed in the field, the sophistication of face models has followed. Earlier face model designs for laboratory testing were hard, flat discs, which have subsequently evolved to become more realistic and representative of specific patient groups. Newer face models that incorporate skin, underlying tissue, and structures that are broadly representative of a range of patient populations have become available over the past few years.
Q: What are the implications of the new chapter for bioequivalence testing of MDIs?
A: The purpose of bioequivalence (BE) testing is to establish that a generic product can be used interchangeably with the reference product. To be fully equivalent, a generic MDI used with a spacer or VHC should be interchangeable with the reference product used with a spacer or VHC.
Because most MDIs will be used with an add-on device by certain patient populations, manufacturers usually recommend use of a specific spacer or VHC for use with a particular inhaler. Indeed, regulatory guidances for Canada and Europe recommend this approach.
Now that methods for assessing the impact of an add-on device have been established, the implication is that there should be two discrete BE studies, one of the MDI alone and one using the designated spacer or VHC. If the add-on device specified with the generic is different from the reference product for some reason, a further comparison is also required.


