A Q&A with Kristian Debus and Prashanth Shankara of CD-adapco
 Q: Doesn’t optimization of drug delivery devices using computer simulation require a lot of time and resources?
Q: Doesn’t optimization of drug delivery devices using computer simulation require a lot of time and resources?
KD: Actually, these days you can optimize an inhaler or nasal spray pump design on any high end desktop computer in just a few days or maybe a week. I remember working with a company on an inhaler design 12 years ago, and at that time running four or five cases took four weeks. Now, we can run 80 cases for the same inhaler over just a weekend on a fairly small computer.
Most people start with just a desktop. My own desktop is about $4,000, and I can do a lot of things with it; but even a small cluster might cost only about $20,000, and they are much easier to set up and use than they used to be. And of course, there are cloud solutions that we can work with, too.
It depends on how complex your problem is. Looking at particle deposition is obviously going to take longer than just analyzing air flow because you are talking about small time steps and small particle sizes, but I would say that to get an initial idea of deposition in the nasal cavity, say, you could get a passive scalar model in about a 2-hour run time. For many people, that’s enough, and you can crank through a lot of simulation very quickly that way.
PS: The difference is that a simulation that might take several days on a high end desktop would probably take only one day on a really small cluster. If you only develop one device a year, though, then maybe you don’t need to invest in a cluster; you can definitely run a high end optimization case on a desktop. You only need a dedicated compute cluster if simulation is a part of your everyday design process.
You don’t need to invest a lot in a software license either; these days you can pay as you go. If you want to run simulations for 3 or 4 weeks out of the year, then you can license the software for only those 3 or 4 weeks. Cloud licensing has really opened up accessibility of simulation to a wide variety of users.
For smaller organizations, they might even consider having engineering services run the simulations for them, and just pay by the hour. The service can simulate the problem for you, do the optimization, and hand you a final design before you even build a prototype.
We have cut down design time in other applications like airplanes and Formula 1 race cars from months to a matter of days, and inhalers are much smaller devices with comparatively much easier geometry. So when it comes to optimization, it can easily be done in a day or two with enough computational resources. Of course, the more complex your simulation is, the more time and resources you need.
Q: If you want to run the simulation yourself, what kind of background and training do you need?
KD: It helps to have an engineering background so that you already know the physics and only need to learn to use the tool. If you are doing a basic flow CFD, I think we recommend three days of training, and then you’re pretty much off the ground running with your product.
PS: If you are an engineer and know your product, a few days of training is all that you need, especially if you have a dedicated support engineer to call on for help.
Q: What’s the biggest obstacle to OINDP developers’ use of computer simulation?
PS: At RDD this year, I spoke to to a lot of device developers who weren’t even aware that simulation was available to them. People in the life sciences tend to know that simulation is being used successfully in industries like automotive and aerospace and the biggest challenge can be to convince them that a) there’s value in it; b) it’s not rocket science; and c) it’s affordable and accessible.
Often, the device designer/engineer/developer can see the value of simulation from a technical background but convincing upper management that investing in simulation can save time/cost and improve designs is a challenge. Even though it makes sense from a technical standpoint, the business benefits need to be conveyed to upper management properly, especially in the OINDP industry where simulation is still at a nascent stage.
Q: Beyond optimization of a device, how else can OINDP developers use simulation?
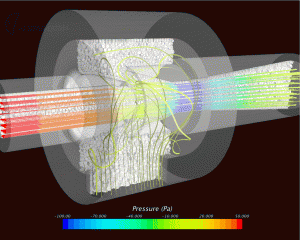
KD: Most people in the industry are using simulation for relatively simple air flow or for deposition modeling down to the lungs. They may also simulate aerosol generation to optimize droplet size distribution in a nasal spray, for example.
Much more complex modeling is possible. In other areas of life sciences, we do a lot of chemistry, things like fermentation in mixing reactors, kinetic reactions, etc. We could be looking at things like mucus formation, the interaction of drugs with the mucus layer, and even drug absorption into the blood vessels.
Usually people break up the models, so for a DPI, they would look at either the inhaler or the aerosol generation or deposition in the lungs or even the mixing of the powder formulation up front; but in other industries, we see a lot that people are starting to stick the various pieces together. It’s definitely possible to model all of those aspects of the inhaler in one big simulation.
Individual patient modeling is another possibility. For example, we can model differences in air flow and deposition in pediatric or geriatric patients compared to adult patients. We’ve worked with researchers who have shown differences from one side of the nose to the other in individuals. And we have clinicians looking to model air flow in the nasal cavity pre- and post-operatively in surgical patients, which could have potential applications for clinical studies.
Some academics are also doing extremely detailed air flow studies; for example, researchers at Imperial College London have done extensive simulation of the flow in the nasal cavity, resolving the flow very finely using a direct numerical simulation. It’s the best you can do to really capture all the flow physics and resolve all the little vortices and eddies and turbulence, but it takes a long time on a very large computer. The idea is to start with that type of very fine model and then make it coarser and coarser so that it’s practical for things like inhaled drug studies.

Kristian Debus is the Director of Life Sciences at CD-adapco

Prashanth Shankara is a Senior Technical Marketing Engineer at CD-adapco


